The U.S. Food and Drug Administration (FDA) has issued a draft guidance intended to assist cosmetics companies in submitting facility registrations, product listings, and ingredient lists to the agency. This guidance precedes the mandatory implementation of the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) later this year. MoCRA is, according to the FDA, “the most significant expansion of FDA’s authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic (FD&C) Act was passed in 1938.”
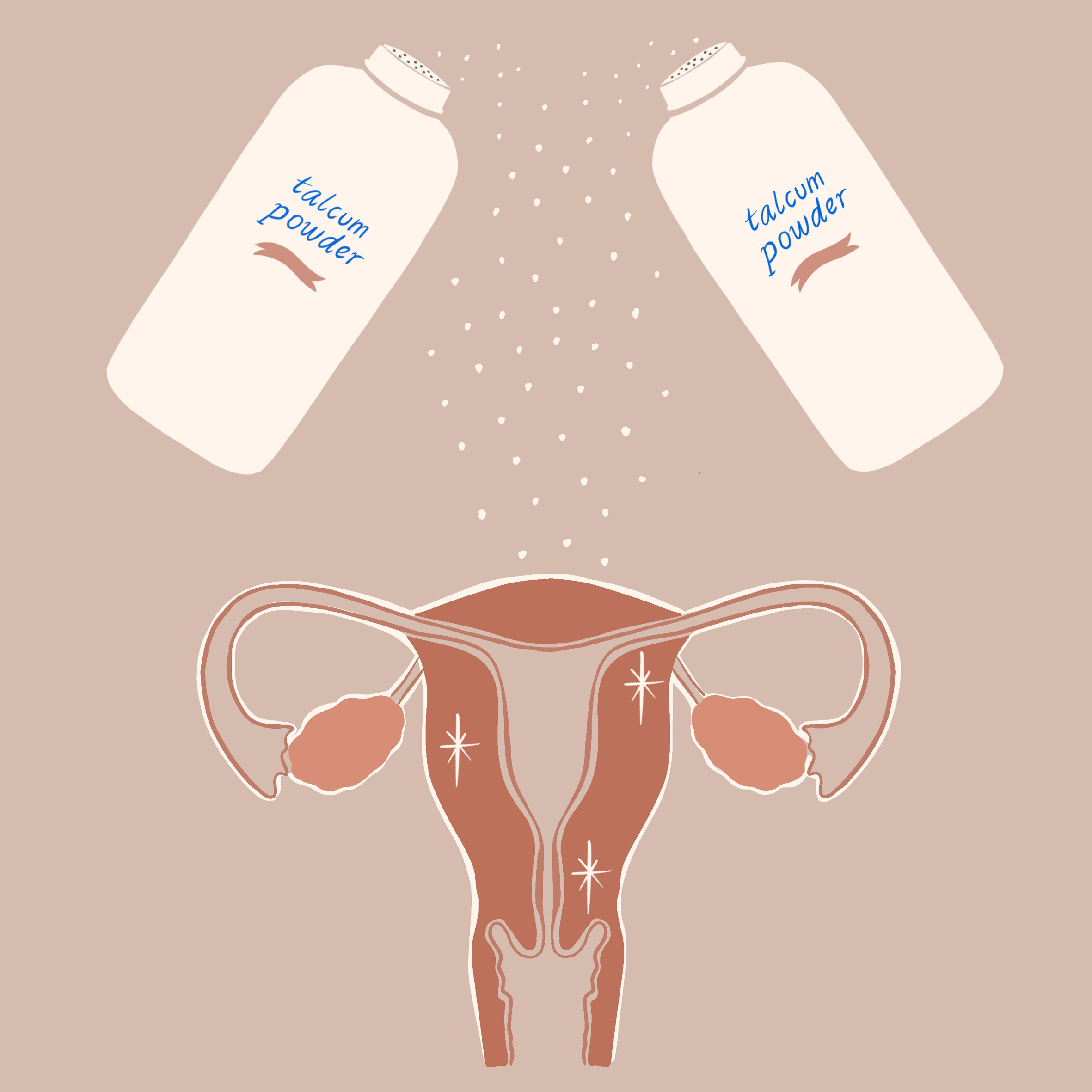
With the average American using between 6 and 12 cosmetic products every day, MoCRA was deemed a vital safety measure for consumer health. In the past few years, many cosmetic products have been called into question after impurities were discovered during routine testing. Some notable examples include talcum powder-based cosmetics products sold at the children’s retailer Claire’s and products manufactured by Johnson & Johnson that were both found to contain asbestos.
With the implementation of MoCRA, the manufacturers of these and other cosmetic products will be required to provide extensive lists of information to the FDA that will allow the agency to detect these deficiencies earlier, pull harmful products from shelves faster, and overall institute a safer cosmetic space for consumers.
Under MoCRA, cosmetics manufacturers will have until December 29 to submit all information pertaining to the cosmetics that a company is producing, what ingredients are in each product, and where the products are being produced. The list of affected cosmetics includes several daily-use products such as:
- Face and body cleansers
- Hair care products
- Moisturizer
- Nail polish
- Perfume
- Shaving cream
- Skincare products
Many of these products have been subject to recalls but have not had preventative measures in place to stop unsafe products from hitting shelves. According to Namandjé Bumpus, Ph.D., FDA’s Chief Scientist, “Until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced.”
If you or a loved one have been harmed by unsafe cosmetic products, you may be able to recover financial compensation. Contact MedTruth today for a free consultation. Our staff wants to get you the just outcome you deserve, and we prioritize the client experience above all else.