Talcum Powder
Legal Developments
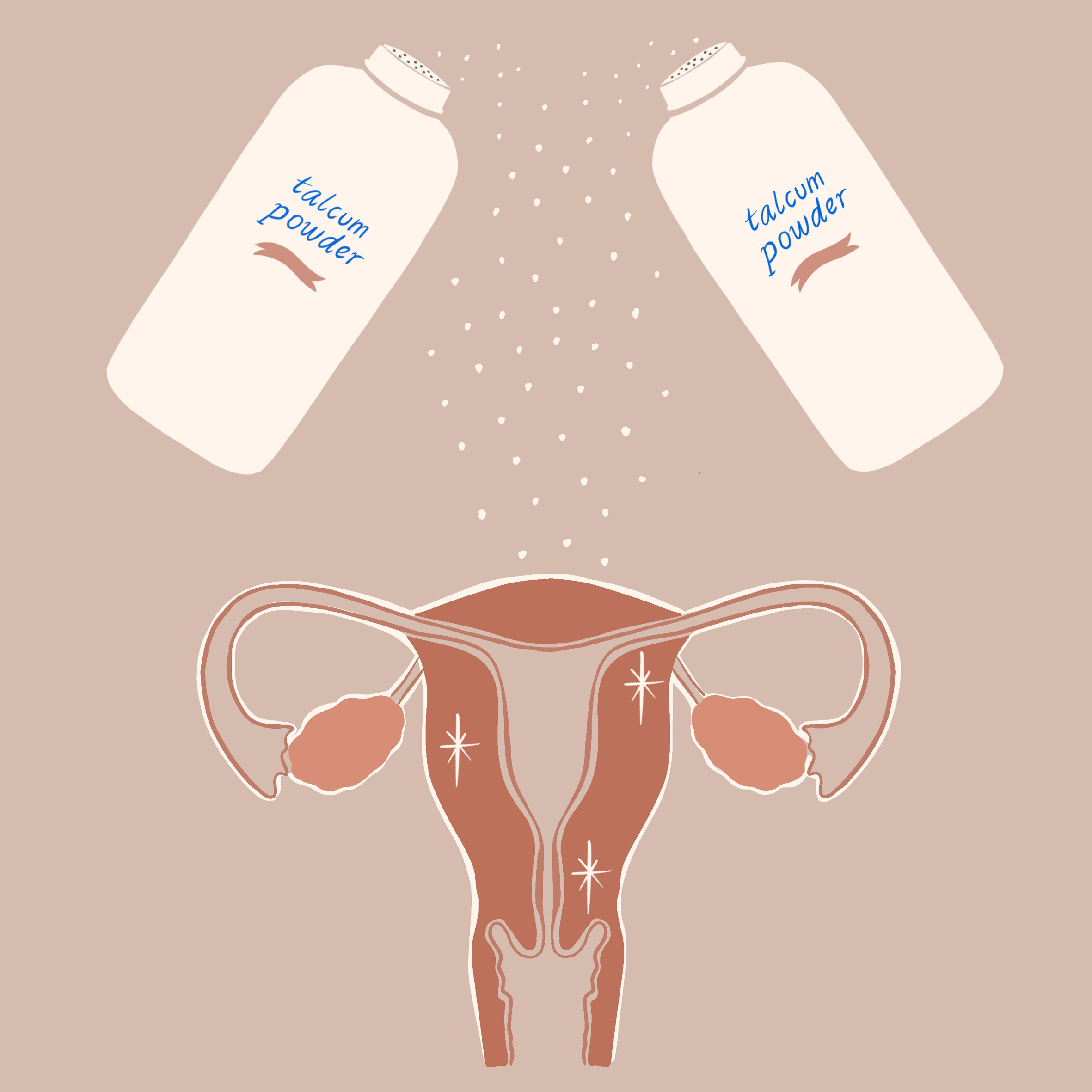
Johnson & Johnson's Talc Litigation Continues
Johnson & Johnson (J&J) has received a new trial in the case of a $417 million verdict with a woman who claimed that their baby powder gave her ovarian cancer.
The California appeals court sustains the lawsuit’s first assertion that Johnson&Johnson failed to properly warn Eva Echeverria about the health risks of talc-based powder. Unfortunately for Ms. Echeverria, who died approximately a month after the jury delivered its August 2017 decision, the judge did not agree with the lawsuit’s second assertion that talcum powder may cause ovarian cancer. Johnson&Johnson spokesman Ernie Knewitz has stated that he is looking forward to retrying the second assertion. Johnson&Johnson has already paid out over $330 million in June for claims blaming talcum powder for giving consumers cancer, and they hope to turn around their recent losing streak in this retrial. Echevveria's daughter, Elisha, awaits the retrial as as trustee and, now, named plaintiff.
HIV
Studies
Seven-Year Grant to Fund Innovations in HIV Treatment
The National Institute of Health has announced earlier this month that they are committing $129 million to HIV research led by Scripps Research.
The seven-year grant intends to continue the work that has been conducted with the previous seven-year grant and begin human trials for HIV treatment. Other companies, including British multinational company GlaxoSmithKline, have also been striving to find successful treatment methods for HIV. Their product, Dovato, was able to suppress the disease for 48 weeks in a recent trial that ended last Wednesday. Australia has made similar strides by instructing citizens in the use of the PrEP drug regimen which has been shown to drastically reduce the contraction rate of HIV.
Zofran
Legal Developments
GlaxoSmithKline Requests an FDA Statement on Zofran Birth Defects Litigation
GlaxoSmithKline (GSK) requested that the U.S. Food and Drug Administration (FDA) release a statement reporting the claims of hundreds of Zofran lawsuits against them.
GSK argues they cannot be held liable for birth defects caused after pregnant women were prescribed the drug because the FDA rejected any scientific correlation between the medication and health risks. GSK representation contends that the FDA twice considered whether or not to add pregnancy warnings to the Zofran label and declined both times.
Adamant that this information will sway the court in their favor, GSK has submitted a motion for U.S. District Judge F. Dennis Saylor to delay the first Zofran trial until the FDA releases a formal statement on the Zofran lawsuits. This statement would address whether the FDA believes GSK failed to warn consumers of Zofran's risks, whether the studies cited in the litigation would warrant a labeling change, and whether the FDA believes federal law preempts the state laws plaintiffs are using to file the lawsuits. As of this time, the FDA has not commented.
Opioids
Public Health
Focus Shifts from Overprescription to Institutionalized Addiction
In a federal report released Wednesday, promising numbers were released amid the opioid crisis. According to a report on Medicare beneficiaries provided by Kaiser Health News:
- Prescriptions for opioids through Medicare are down by 11%
- The number of beneficiaries at risk for overdose is down by 46%
- The number of doctors prescribing opioids to at-risk patients is down by 51%
While the numbers seem to be decreasing, the University of Michigan discovered on Thursday that the number of opioids being prescribed for minor injuries, such as a sprained ankle, is becoming uncommonly high. These conflicting statements go to the heart of the issue’s complexity, as explored by the New York Times.
For many, the opioid crisis’ first wave of ignorance and overprescription has ended. Now, the crisis has entered a stage of institutionalized addiction and needless use that must be treated.
Breast Implants
Regulation
Breast Implants Banned in Australia
Though the U.S. Food and Drug Administration (FDA) has yet to conclusively state whether textured breast implants place women’s health at risk, individuals and organizations across the world are taking action.
In Florida, Dr. Fredric Barr has stated that he will no longer be offering breast implant surgeries to women until the FDA and scientists have more answers about implant-related illnesses including breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). While these concerns remain unanswered in America, Australia's Therapeutic Goods Administration and Health Minister Greg Hunt have proposed to ban or suspend 25 models of breast implants due to their association with elevated risks of a rare cancer of the immune system.