Cancer patients taking the oral chemotherapy drug Tasigna should be aware that they’re at risk of developing peripheral neuropathy.
Unfortunately, the condition is not mentioned as a side effect on the patient website of Swiss drug manufacturer Novartis.
Chemotherapy-Induced Peripheral Neuropathy
Peripheral neuropathy is an umbrella term referring to many conditions that involve damage to the peripheral nerves, a vast anatomical communication network that sends messages back and forth between the brain and spinal cord, or central nervous system, and the rest of the body.
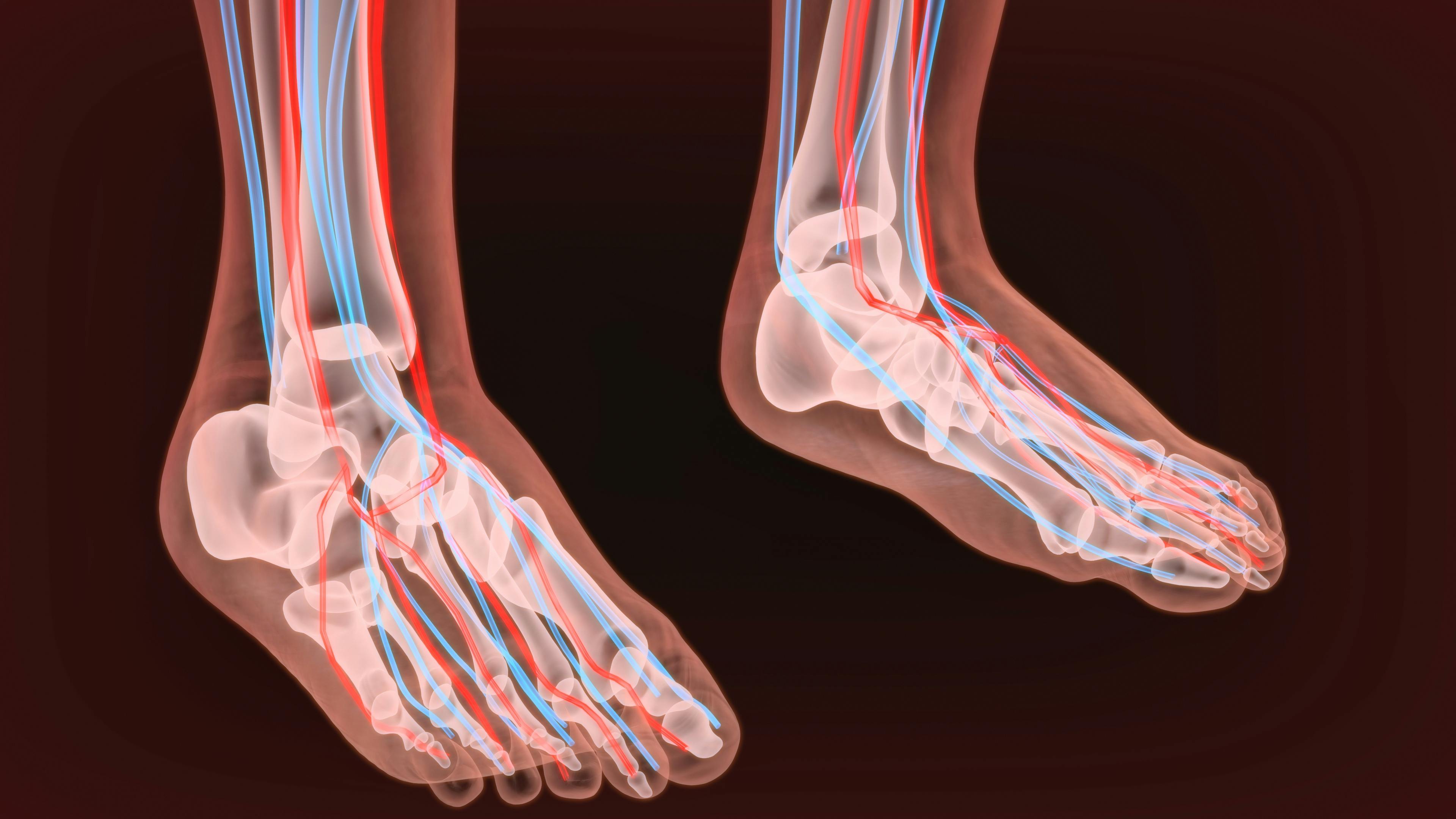
Chemotherapy is one cause of peripheral neuropathy. Other causes include diabetes, physical damage to the nerves, vascular and blood problems, hormonal and nutritional imbalances, exposure to heavy metals or toxic chemicals, infections, kidney and liver problems, and some cancers and benign tumors.
It’s estimated that approximately 30-40 percent of chemotherapy patients experience chemotherapy-induced peripheral neuropathy (CIPN). For patients taking chemotherapy drugs such as Tasigna, peripheral neuropathy symptoms, unfortunately, can persist for a long time after discontinuing chemotherapy.
Symptoms of Peripheral Neuropathy
Symptoms of peripheral neuropathy vary depending on which types of peripheral nerves are affected: motor nerves that control muscle movement, sensory nerves that receive sense impressions, or autonomic nerves, which control respiration, digestion, blood pressure and other physiological processes. The condition can range from mild to severe.
Symptoms when motor and/or sensory nerves are affected include muscle weakness, numbness, tingling or pain in the hands and feet. Pain can take the form of burning, freezing, tingling or stabbing sensations. Symptoms can spread to the arms and legs as well as other areas of the body. Cramping, twitching and muscle shrinkage can also occur, as can extreme sensitivity to touch and lack of coordination and falling.
Symptoms when the autonomic nerves are affected may include heat intolerance and changes in sweating patterns, bowel, bladder or digestive problems, and dizziness and lightheadedness from blood pressure issues.
How to Reduce the Risk of CIPN
Treatments used prevent CIPN include both supplements – vitamin E, calcium and magnesium, and glutathione, an enzyme and important antioxidant in the body, and pharmaceuticals – antidepressants such as Effexor and anti-seizure medications such as Tegretol. Other vitamins, dietary supplements and pharmaceuticals are currently being studied as preventative treatments, but at present, there’s no definitive way to prevent CIPN.
Chemotherapy can be administered in ways that may reduce the risk of CIPN. These strategies include breaking up a single large dose into several smaller doses, spreading doses out over a longer time frame, or reducing the dosage to a point where most of the benefits are still retained.
Tasigna: Chemo for a Rare Form of Leukemia
Tasigna, known generically as nilotinib, was first approved by the FDA in 2007 to treat a rare form of cancer known as Philadelphia positive–chronic myelogenous leukemia (Ph+ CML). The vast majority of patients with CML, also known as chronic myeloid leukemia, have the “Philadelphia Chromosome” and the Ph+ CML version of the disease. Ph+ is discovered during routine bloodwork and indicates a much greater risk of developing Ph+ CML.
About Chronic Myelogenous Leukemia
CML represents about 10 percent of all leukemia and 3 percent of childhood leukemia diagnoses. In a given year, approximately 9,000 Americans were expected to be diagnosed with the disease and about 1,800 to die from it. CML is generally diagnosed in individuals age 64 or older, with about 60 percent of those diagnosed are male.
Tasigna is considered a “second line” therapy for patients who are unable to tolerate or otherwise fail on chemo drugs such as imatinib, generic for Gleevec, also manufactured by Novartis. In the United States, Tasigna is approved for use in both adults and children (one year of age or older) who are newly diagnosed with the disease as well as for those in the chronic stage. In March, the FDA approved Tasigna as a treatment for chronic phase CML in children one year and older.
CML occurs when genetic changes take place in the myeloid cells, bone marrow cells that produce red blood cells, platelets, and most forms of white blood cells. The Philadelphia Chromosome produces BCR-ABL, a protein that changes the blood-producing myeloid cells into abnormally-growing CML cells.
Other Tasigna Side Effects
Other side effects of Tasigna include allergic reactions, serious heart problems such as rapid pounding and sudden dizziness, liver and pancreas problems, shortness of breath, unusual bruising and bleeding – including bleeding in the brain, sudden weight gain, low blood cell counts, and severe peripheral occlusive arterial disease. This is a partial list.