Prescription Dependency
Regulations
DEA Initiates Drug Take-Back Program to Curb Addiction
A concerted effort to minimize prescription drug addiction is taking place nationwide this weekend, October 28.
The Drug Enforcement Association (DEA) initiated the National Prescription Drug Take-Back program 14 years ago as a way to “offer the public a safe and convenient way to get rid of pills that, if abused, can lead to addiction.”
According to the U.S. Centers for Disease Control and Prevention, about 91 Americans die from a prescription drug overdose every day. It’s alarmingly easy to procure prescription medication from the home of a friend or family member, and simply throwing them away or flushing them down the toilet presents health and safety hazards.
You can find more information, as well as designated collection sites on the DEA website.
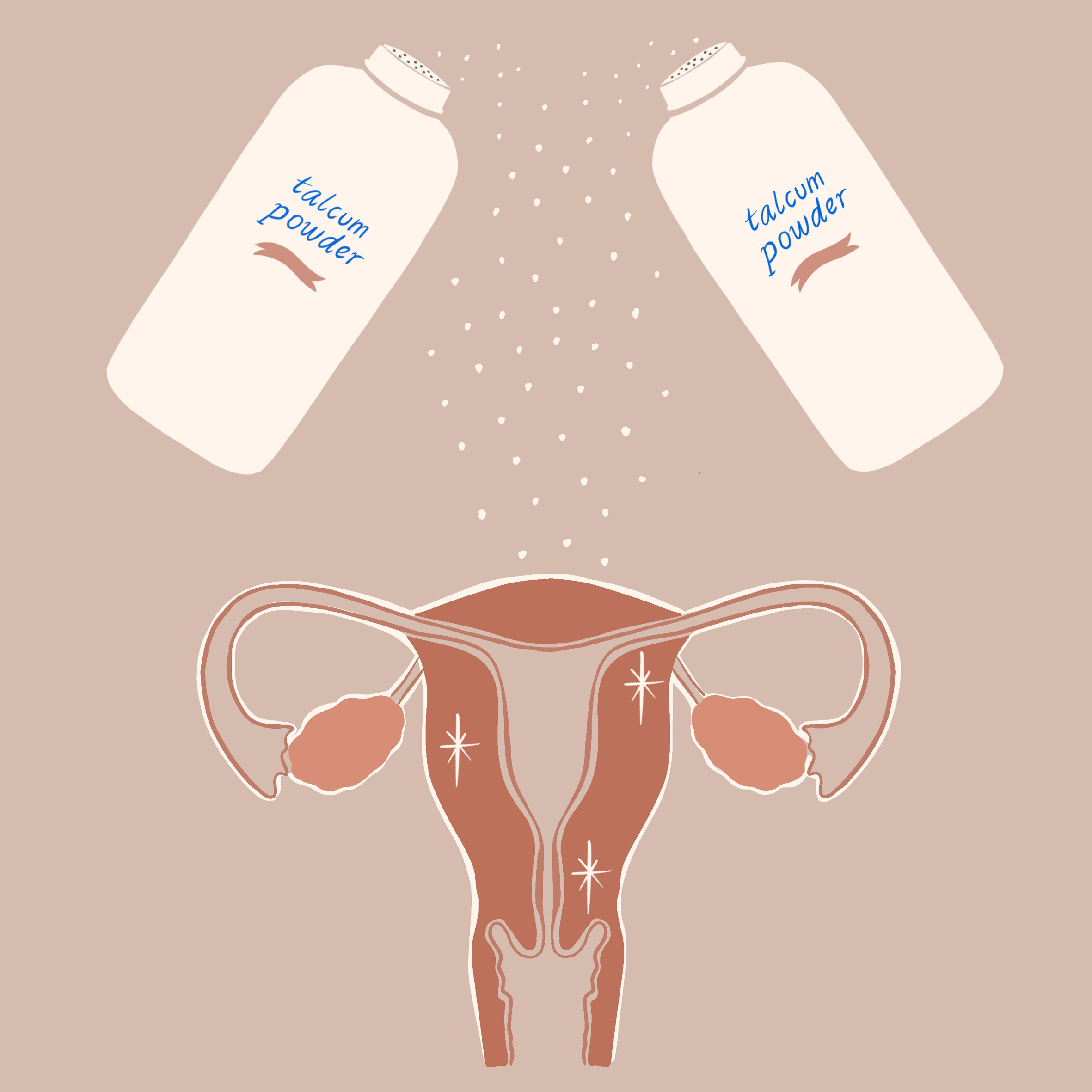
Talcum Powder
Advocacy
Verdict Reversals in Talcum Lawsuits Spell Victory for J & J
In a stunning upset to ovarian cancer advocacy, Los Angeles County Superior Court Judge Maren Nelson reversed the verdict that would have awarded $417 million to plaintiff Eva Echeverria. Judge Nelson cited "insufficiency of evidence" for her claim and excessive damages award.
The California resident initially won her suit against Johnson & Johnson in August after claiming the prolonged use of Johnson’s Baby Powder may have caused her to develop ovarian cancer. The second such reversal in a week, plaintiff and Alabama resident Jacqueline Fox also had her $72 million award against J & J rescinded. According to ABC News, "The appeals court cited a Supreme Court ruling in June that placed limits on where injury lawsuits could be filed, saying state courts cannot hear claims against companies not based in the state where alleged injuries occurred."
Approximately 4,800 legal cases against J & J are still pending amidst continuing scientific investigation as to the effects of long-term talc use and its links to ovarian cancer. In the meantime, Echeverria’s lawyers have begun filing an appeal against the verdict reversal.
Opioid Epidemic
News
Esquire and The New Yorker Break The Opioid Epidemic Wide Open
There is finally some light being cast on the dark shadow of opioid dependency. Both Esquire, and The New Yorker have published extensive write-ups focusing on the severe damage caused by OxyContin addiction, tracing the origins of the Sackler family and its management of a national crisis.
Both articles by Esquire and The New Yorker present confronting portraits of a nation struggling under the oppressive weight of opioid addiction, from the sales team who thought they were helping people with pain relief, to the parents planning funerals for their children who died from an overdose or adverse reaction.
On October 26, President Trump signed a memorandum, stating in a press briefing: “Effective today, my Administration is officially declaring the opioid crisis a national public health emergency under federal law.”
Opioids now kill more than 50,000 Americans each year, a greater number than gun-related homicides and vehicular deaths.
Vaginal Mesh
Devices Abroad
J & J Facing Class Action Lawsuit Over Flawed Vaginal Mesh
Debilitating pain and other adverse effects have caused more than 700 Australian women to file a class action lawsuit against Johnson & Johnson over flawed vaginal mesh implants. The mesh was designed to aid with stress urinary incontinence and pelvic organ prolapse, complications that may arise following childbirth.
Mesh erosion is the most common adverse effect, wherein the mesh migrates and erodes into either the bladder or bowel, causing pain, bleeding, and in the most severe cases, additional surgery.
Lawsuits related to mesh implants have also been filed in the U.S. and U.K. against Johnson & Johnson and other manufacturers. One U.S. woman was awarded $57 million for damages from a Johnson & Johnson mesh product.
Online Prescriptions
Pharma Trends
A Warning About Online Pharmacies
The Federal Drug Administration (FDA) has publicly warned of the dangers of online prescription medication purchases. Online pharmacies have fast become an option for those wanting to avoid escalating prescription drug costs.
According to Consumer Affairs, “National Association of Boards of Pharmacy, an industry trade group, issued a report containing a review of more than 100 websites that were said to be operating from Canada.” The report found that the majority were not actually based in Canada, and no valid prescription was required to make a purchase.
The FDA has stressed proceeding with caution, since “the only way to know if you are dealing with a safe and legal online pharmacy is to use your state board of pharmacy’s license database via an online tool,” which requires a valid doctor’s prescription, and a valid U.S. phone number and address.
Is there a medical topic or trend you'd like us to talk about? Let us know at info@medtruth.com.