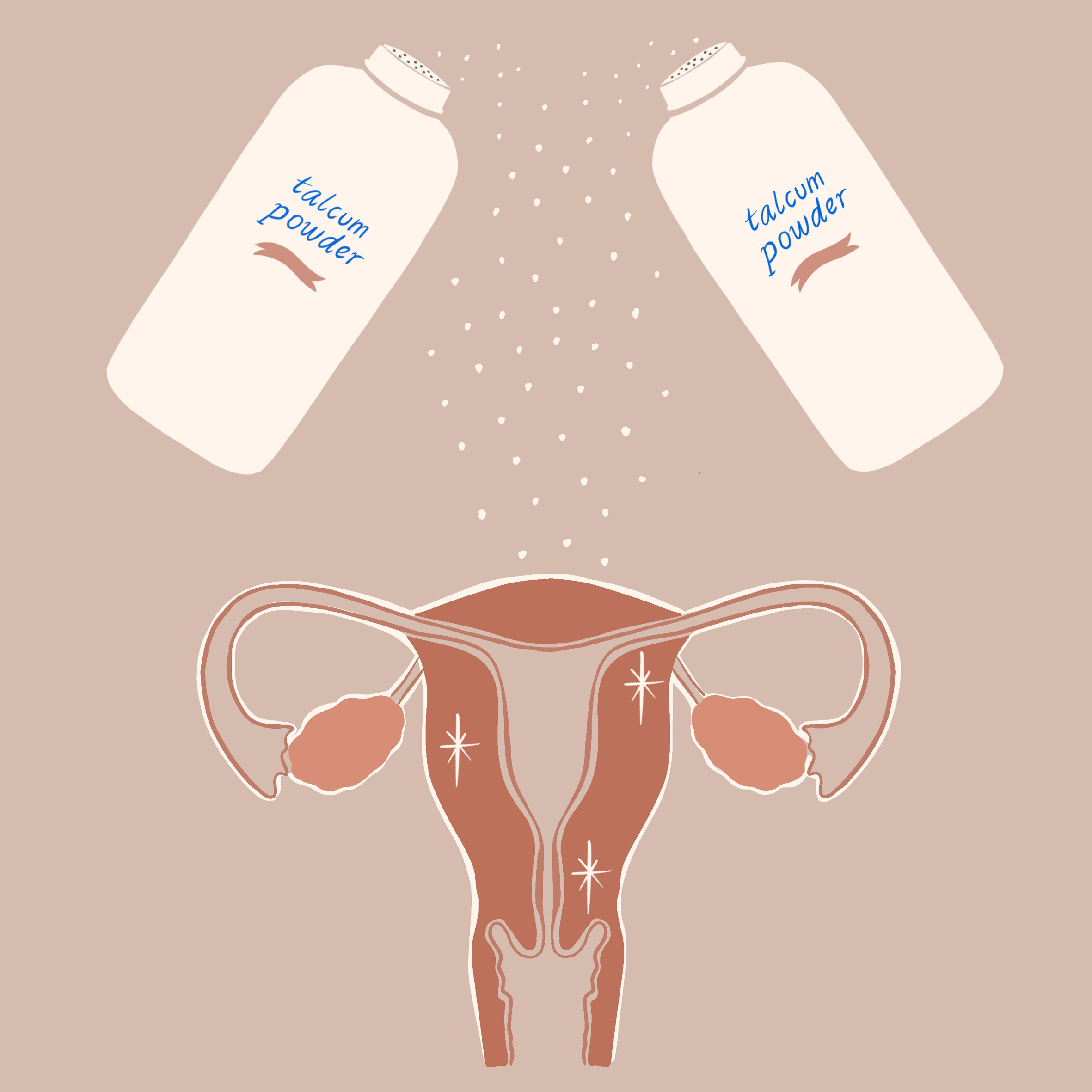
Global health and personal care products giant Johnson & Johnson is discontinuing sales of its talc-based baby powder in the United States and Canada, multiple news outlets including The New York Times, The Wall Street Journal, Bloomberg, and CNBC announced Tuesday afternoon. The company has been selling its once iconic, now controversial, product for 127 years, according to Bloomberg.
Since 2014, Johnson and Johnson has faced nearly 20,000 lawsuits alleging that asbestos-contaminated talc in its baby powder caused ovarian cancer or mesothelioma, a cancer of the lining of the lungs, in plaintiffs. (Asbestos has also been linked to cancer of the larynx.)
Asbestos is a naturally-occurring but carcinogenic mineral often found in close proximity to talc, which is mined.
Johnson and Johnson will continue selling a cornstarch-based version of its baby powder, which according to Kathleen Widmer, chairman of the company’s North America consumer unit, represents 75% of the company’s U.S. baby powder sales, as reported by Bloomberg. Sales of talc-based baby powder will continue until supplies are exhausted. The company began selling the cornstarch version in 1980.
CNBC reported that Johnson & Johnson continues to assert the safety of its talcum-based baby powder but is facing declining sales “fueled by misinformation around the safety of the product and a constant barrage of litigation advertising.”
In October, the company voluntarily recalled 33,000 bottles of its talcum-based baby powder after the U.S. Food and Drug Administration found sub-trace amounts of asbestos in a single bottle purchased online.